Abstract
Introduction: Patients with R/R CD30+ malignancies such as PTCL, CTCL and cHL often lack effective and tolerable therapy options. Available treatments frequently provide only short-term disease control at the cost of significant or cumulative toxicities. Brentuximab-Vedotin (BV, Adcetris®), an MMAE-delivering anti-CD30-ADC, has a high response rate but dose-limiting AEs often result in premature dose reduction or even therapy discontinuation. The maleimide chemistry results in a heterogeneous product (DAR 0-8) with varying PK and potency properties and spontaneous deconjugation can cause premature transfer of linker payload to serum proteins and thereby reduce the tumor selectivity. In consequence, some of the BV-induced toxicity is likely design-based leading to CD30-independent cellular uptake and aggregation of the ADC.
Objectives: We aimed to generate a novel CD30-ADC with improved biophysical properties to widen the therapeutic window using Tub-tag-conjugation technology.
Material & methods: TUB-010 is a next-generation ADC, implementing a novel conjugation strategy, called Tub-tag® conjugation (Schumacher, Angewandte Chemie 2015). The recombinant Tub-tag sequence, a 14 amino acid peptide derived from α-tubulin and fused to the C-termini of the CD30 mAb light chains, has unique features in that it provides a significant degree of hydrophilicity to counterbalance the payload-derived hydrophobicity, thus enabling superior biophysical properties and product homogeneity. This new technology was used to generate a DAR2-ADC with stably linked MMAE via a Cathepsin B cleavable linker (vc-PAB) and chemoenzymatic conjugation of linker payload using Tubulin-tyrosine ligase (TTL). The resulting ADC was tested for physicochemical properties, homogeneity, stability, internalization, preclinical efficacy, nonspecific uptake and general cytotoxicity in primary cells, cell lines, rats & cynomolgus monkeys.
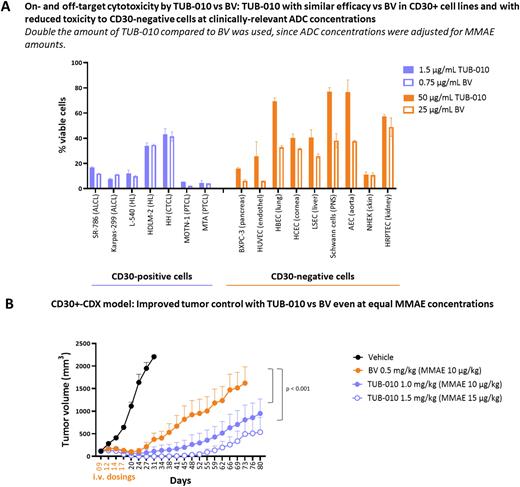
Results: Tub-tag technology enabled us to create a DAR2 anti-CD30-ADC with a binding affinity, internalization and lysosomal release characteristics similar to BV. In contrast to BV, TUB-010 is a homogeneous DAR2 ADC. The linker-payload is chemically stably attached to the mAb with neglectable premature deconjugation while in circulation, whereas protease-mediated release of MMAE within the lysosome occurs normally as expected with the vc-PAB linker. The increased ADC stability prior to internalization via CD30 leads to reduced nonspecific transfer of linker-payload to serum proteins, and overall improved PK parameters in rats. The hydrophobicity of MMAE is counterbalanced by hydrophilicity provided by the Tub-tag peptides, which resulted in > 5-fold reduced ADC aggregation under stress conditions compared to BV. The increased hydrophilicity of the overall ADC reduced nonspecific cellular uptake and consecutive cellular toxicity vs BV within the clinically therapeutic range by factor 2- to >10-fold in various human primary cells (Fig. A).
When normalized to the MMAE concentration, TUB-010 showed similar in vitro cytotoxic efficacy as well as similar bystander activity compared to BV on established cancer cell lines from cHL, PTCL and CTCL patients with various levels of CD30 expression (Fig. A). In vivo, TUB-010 exerted significantly improved tumor control compared to BV when both ADCs were dosed at equal MMAE concentration in the Karpas-299 cell-derived ALCL xenograft model (Fig. B). In cynomolgus monkey dose range finding and GLP toxicity studies, TUB-010 administered 2 or 4 times Q3W in a dose range of 6-15 mg/kg showed a 5-fold widened therapeutic window for the ADC as the MTD was not reached. Hematological toxicity was reduced compared to BV and no signs of anatomical of functional tissue damage were detectable.
Conclusions: Tub-tag technology allows the reduction of nonspecific target-independent toxicity of ADCs in preclinical models by improving key design issues in current ADCs such as stability and overall biophysical features. TUB-010 is a promising, novel and potential best-in-class CD30-ADC which can deliver the cytotoxic payload to CD30+ malignancies with higher precision and with a wider therapeutic window than BV. These features suggest that TUB-010 may increase the clinical benefit with CD30-targeting ADC therapy for patients with CD30+ malignancies.
Disclosures
Gerlach:Tubulis GmbH: Current Employment. Schmitt:Tubulis GmbH: Current Employment. Cyprys:Tubulis GmbH: Current Employment. Kasper:Tubulis GmbH: Current Employment. Mai:Tubulis GmbH: Current Employment. Vermeer:Kyowa Kirin, Recordati: Honoraria, Research Funding; Tubulis GmbH: Consultancy. Horwitz:Acrotech, Affimed, Cimeio Therapeutics, Daiichi Sankyo, Kura Oncology, Kyowa Hakko Kirin, Myeloid Therapeutics, ONO Pharmaceuticals, Secura Bio, Shoreline Biosciences, Takeda, Trillium, Tubulis, Vividion Therapeutics, and Yingli Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics, Affimed, C4 Therapeutics, Celgene, Crispr Therapeutics, Daiichi Sankyo, Kyowa Hakko Kirin, Millennium/Takeda, Seattle Genetics, and Verastem/Secura Bio: Research Funding. Fingerle-Rowson:Tubulis GmbH: Current Employment; MorphoSys: Ended employment in the past 24 months. Vogl:Tubulis GmbH: Current Employment. Schumacher:Tubulis GmbH: Current Employment, Current equity holder in private company. Helma-Smets:Tubulis GmbH: Current Employment, Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.